Today is Christmas Eve, and we have reached the end of our #InvertebrateCalendar – thank you so much to all of our contributors, and to you readers!
We hope you have enjoyed it!
To all of you from all of us:

Today is Christmas Eve, and we have reached the end of our #InvertebrateCalendar – thank you so much to all of our contributors, and to you readers!
We hope you have enjoyed it!
To all of you from all of us:

Hyalinoecia tubicola from the North Sea (by K. Kongshavn).
Quill worms belong to the annelid family Onuphidae and are called like that because of their unique tubes. The tubes are secreted by their inhabitants and are very light and rigid, resembling a quill, the basal part of a bird’s feather used for writing. Quill worms are epibenthic creatures capable of crawling on the surface of the sea floor carrying their tubes along. Their anterior feet are modified, strengthened and enlarged, bearing thick and stout bristles. These anterior feet are used for locomotion.
Quill worms are widely distributed in the ocean inhabiting mostly slope depths down to 2000 m. Being large in body size (up to 10-20 cm long), they can be quite abundant in some areas. Meyer et al. (2016) reported Hyalinoecia artifex reaching up to 70 ind./m2 in the Baltimore Canyon at 400 m water depth. Another quill worm, H. tubicola, which is very common in Norwegian waters, reached up to 272 ind./m2 at 365 m offshore of Chesapeake Bay (Wigley & Emery 1967).
Quill worms are believed to be motile scavengers. Baited monster camera experiments performed at 2000 m deep site in Baja California demonstrated that Hyalinoecia worms can accumulate in hundreds of specimens five hours after the bait (rotten fish) has been deployed (Dayton & Hessler 1972). Myer et al. (2016) analyzed the stable isotope content in Hyalinoecia artifex tissues confirming its secondary consumer status. Their results supported earlier observations on the gut content of the same species by Gaston (1987) showing the presence of the remains of various benthic invertebrates.
Video 1. Quill worm Hyalinoecia tubicola moving inside its tube (by K. Kongshavn).
Video 2. Quill worm Hyalinoecia tubicola protruding from the tube opening. Three antennae and a pair of palps are seen on the head. The first two pairs of feet are enlarged and strengthened (by K. Kongshavn).
Dayton, P.K., Hessler, R.R., 1972. Role of biological disturbance in maintaining diversity in the deep sea. Deep-Sea Research 19: 199–208.
Meyer, K.S., Wagner, J.K.S., Ball, B., Turner, P.J., Young, C.M., Van Dover, C.L. 2016. Hyalinoecia artifex: Field notes on a charismatic and abundant epifaunal polychaete on the US Atlantic continental margin. Invertebrate Biology 135: 211–224. doi:10.1111/ivb.12132
Gaston, G.R. 1987. Benthic polychaeta of the Middle Atlantic Bight: feeding and distribution. Marine Ecology Progress Series 36: 251–262.
Wigley, R.L., Emery, K.O. 1967. Benthic animals, particularly Hyalinoecia (Annelida) and Ophiomusium (Echinodermata), in sea-bottom photographs from the continental slope. In: Deep-Sea Photography. Hersey JB, ed., pp. 235–250. John Hopkins Press, Baltimore.
-Nataliya
At first glance, it can look like a seaweed. The depth, however, should start your alarm-bells for flora and point you towards fauna: the plantlike animal Securiflustra securifrons (Pallas, 1766) is a bryozoa – a collection of colonial filterfeeders less than 1 mm in size each. We are at 80-120 m depth in the cold Heleysundet – the sound between the two islands Spitsbergen and Barents Island in the eastern part of the Svalbard Archipelago. This is a sound famous among captains for its fast tidal streams, and the fast-flowing waters give the bryozoans a nice place to live. The colonies branch out to catch the most water-flow and the most food from the water.
Where the “branches” form we see what might look like small hairy balls – these are the bivalve Musculus discors (L., 1767). The hairy look comes from their byssus threads – they produce and then use these threads to attach to the Securiflustra (and being packed in the threads they might get some camouflage from them).
Moving inside the molluscs we might find not only one, but two species of amphipods. In our samples from Heleysundet 14% of the Musculus had the carnivorous amphipod Anonyx nugax Ohlin, 1895 inside, and an astonishing 3 out of 4 Musculus had amphipods of the species Metopa glacialis (Krøyer, 1842) inside. The system resembles a Russian doll – one species living inside another living inside yet another…

Anonyx affinis (large amphipod, upper left) and Metopa glacialis (small amphipod lower half og mussel) innside a Musculus discors. Photo: AHS Tandberg
What reason can a small crustacean have to live inside the quite closed off world of a bivalve? The bivalve filters water actively – it pumps water over its gills, and then transports food-particles such as phytoplankton down the gills towards its mouth. Non-desirable particles are normally packed into mucus and transported out of the bivalve. Now imagine liking to eat some of those particles the bivalve finds non-desirable, and being placed on the gills of said bivalve. No need to hunt for the food – it will be coming on the conveyor-belt the gills are – and all you need to do is to eat. The bivalve does not seem to be troubled by this co-habitant – it does not eat the same food as the bivalve.
Not only does Musculus discors provide Metopa glacialis with food, the mantle cavity provides a luxury-shelter where the amphipod can raise a family! Amphipods, together with isopods, cumaceans, tanaidaeans and quite a few mysicadeans keep their offspring in a brood-pouch from the fertilisation of the eggs to the medium sized juveniles crawl out into the real world. Living inside a bivalve allows Metopa glacials to extend its child-care to young life outside the brood-pouch. Our examinations of the bivalves from Heleysundet showed us adult Metopa in the middle of the bivalve, with several juveniles “strategically placed” inbetween the two layers of gills in each shell-half. Surrounded by food, safe from most predators! (Predation of Metopa glacialis might be the main objective for Anonyx affinis, the food-source of the lysianassid needs to be established. It might also be the nice and fatty mollusk.)
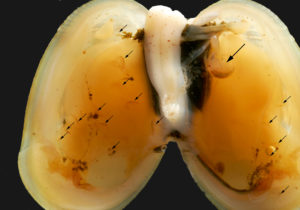
Metopa glacialis innside a Musculus discors. Small arrows point to juveniles, large arrow to adult female. Photo: AHS Tandberg
Comparing with amphipods of the same size-range from the same areas, Metopa glacialis seems to have a safe life. Safe enough that they can manage to have several sets of offspring. We see that they don´t wait until´the first batch of kids are out of the “house” – we found one adult female with two size-groups of offspring and a fresh egg-filled brood-pouch! Each batch can be 20 offspring, so that would mean one pregnant mom and 40 kids in one small house!
Many people travel to visit family during the holidays. Even when we cherish the time with our loved ones, filling the house with grandparents, aunts, uncles and cousins might cramp everybodys style slightly. Not so with Metopa glacialis. Measuring the size of all inhabitants show us that the kids stay home until they are adult and can move out to their own home. So when you can´t sleep because your younger cousin plays on her gamer all night, or because your old aunt snores when you come into your shared room, think how much more difficult life could have been if you were an amphipod. Happy holidays!
Anne Helene
PS: A slightly extended version in Norwegian (part of the TangloppeTorsdag blog) can be read here)
Literature:
Just J (1983) Anonyx affinis (Crust., Amphipoda: Lysianassidae), commensal in the bivalve Musculus laevigatus, with notes on Metopa glacialis (Amphipoda: Stenothoidae). Astarte 12, 69-74
Tandberg AHS, Schander C, Pleijel F (2010) First record of the association between the amphipod Metopa alderii and the bivalve Musculus. Marine Biodiversity Records 3:e5 doi:10.1017/S1755267209991102
Tandberg AHS, Vader W, Berge J (2010) Studies on the association of Metopa glacialis (Amphipoda, Crustacea) and Musculus discors (Mollusca, Mytilidae). Polar Biology 33, 1407-1418
Vader W, Beehler CL (1983) Metopa glacialis (Amphipoda, Stenothoidae) in the Barents and Beaufort Seas, and its association with the lamellibranchs Musculus niger and M. discors s. l. Astarte 12:57–61
“Julebukken” – the Yule Goat.
A goat made of straw has commonly been observed among the paraphernalia that people put on display around Christmas times in the Nordic countries. Ask people what they symbolize and I bet that the majority would say just “Christmas” without having a further explanation at hand. It is very likely that the Yule Goat is a remnant from the pagan celebrations of the December solstice. The mythological origin of the Yule Goat is unclear (see Gunnell 1995). It is probably of mixed origin because cultural evolution is often syncretic, – a blend of beliefs, mythologies, and practices from different sources and “ethno-folkloristic schools of thought”. It has been speculated that the straw figure of the Yule Goat reflects some sort of pagan vegetation god ruling over grain growth and who required particular human attention around the winter solstice.
Others have associated Yule Goat with the Germanic thunder-god Thor, because he used two bucks to pull his chariot over the sky. Thor must have been an environmentally friendly god because these bucks were used as a food resource in Valhalla and if only he took great care to keep the bones and the skin, he could revive fully reincarnated goats the next day. The idea of a resurrected goat was dramatized in folk rituals of Nordic small communities by people who reenacted the death and revival of the Yule Buck in songs and theater performed on house visits by a ragged assembly of masked people. The central character in these folkloristic plays was a person either wrapped in straw or hides and carrying a goat head, sometimes also a hammer (like Thor). Right up to the mid nineteenth century, plays like these were practiced in Scandinavia. The traditions have several characteristics in common with Halloween and the Yule Buck masquerade apparently is a tradition that now seems to be fading and to be replaced by Halloween celebrations. In both cases there is an underlying theme of temporary breakdown and restoration of cosmological and moral order when cyclic time has gone the full circle. A small scale version of this idea is also at work around midnight, – the ghost hour. The Cristian tradition has tended to associate goats with naughty behaviours of all sorts, particularly in terms of sexuality. Goats were also at times associated with mythological creatures like Pan and with the Devil.
The Star Buck
The Babylonians divided the sky in 360 parts, but ancient astronomers also used twelve 30 degrees sectors of the sky to reference the positions of celestial bodies on the ecliptic, – the track that the sun appears to follow through the year. Like the classic analogous clock with twelve sectors marking divisions of the day and night the zodiac is a clock for the earth’s revolution around the sun. Because the rotation axis of the earth is tilted, the sun appears to draw an S-shaped path around the Earth. From a northern perspective the maximum of the path is the summer solstice. The minimum is the winter solstice, when the zenith of the sun is farthest away from the Arctic and the days are shortest on the Northern hemisphere. The exact time for the solstice is not easy to determine, but ancient astronomers found that the winter solstice took place when the sun was in the sector of the star constellation called Capricornus. Claudius Ptolemy, who is known as the prime authority of pre-Copernican cosmological texts, wrote in Book 1 chapter 11 of his very influential Tetrabiblos :
“For the sun turns when he is at the beginning of these signs and reverses his latitudinal progress, causing summer in Cancer and winter in Capricorn.”
Capricornus is Latin for “goat horn” and Capricorn is sometimes depicted as a goat, sometimes as half goat, half fish. Because the “turning point” of the sun at winter solstice was once at an imaginary latitude circle drawn through Capricornus, we say that the sun is turning at the Tropic of Capricorn. However, due to the swaying of the Earth’s rotation axis, winter solstice is no longer in Capricorn and the Tropic of Capricorn has also moved away so that, paradoxically, the Tropic of Capricorn is now passing through Sagittarius . When the Julian calendar took effect 45 years BC the solstice was celebrated on 25th of December, but apparently winter solstice was already about to leave Capricornus in the direction of Sagittarius. Historians of astronomy think that Capricornus was already a marker of seasonal time about 2000 years BC.
Although much speculation has been put forward about the origins of Yule Buck, I suspect that the role of the goat in the sky has been undervalued when trying to understand the conducts and traditions of people in the Nordic countries around the winter solstice. Surely, the teaching of Ptolemy must have diffused somehow to ordinary people during the Medieval Ages. After all, Capricorn was the messenger of a better existence to come, if one could only sustain over the winter.
Resurrecting a goat
Goats that stood model for the Capricorn have been part of the human environments since long before they were painted on cave walls in Ardeche at the foot of the Pyrenees about 30000 years BC. Archaeological material from Jordan indicates that goats were domesticated already 7000 BC as one of the first of the ruminant species. Wild goats are members of the genus Capra and are distributed with several species over the Eurasian and African continents. Most of the wild goats are now regarded as more or less threatened species due to hunting pressure and habitat loss. In Spain the so-called bucardo goat was declared extinct in year 2000, when the last individual was hit by a falling tree in Oresa National Park.
An international group of biotechnologists set up experiments to resurrect the bucardo, which is considered as a subspecies of the Pyrenean Ibex, Capra pyrenaica. It is perhaps not likely that these scientists were inspired by the Nordic myth about Thor and his perpetual buck-goats. Nevertheless, they had already taken skin samples from the last living female the year before she died. With the frozen cells from the skin they had cellular nuclei with goat genome and also a plasma membrane with small amounts of cytoplasma. With a technique similar to the one that was pioneered by the Roslin Institute in Edinburgh to clone the sheep Dolly, they replaced the cell contents of domestic goat eggs with somatic cell material from the dead ibex. Then they implanted the manipulated eggs into many substitute mothers of both domesticated goats and of females of the Spanish ibex. Only one of the embryos survived long enough to be released by cesarean section and it died only a few minutes after birth. Despite this and similar failed efforts to reconstruct extinct evolutionary lineages, it is still claimed by scientists who are involved in this business that such methods hold promise for rescuing rare and endangered species. But without making claims of being a specialist in conservation genetics I ask myself: how is it possible to save a species when all of the genetic variability in the population is lost. This may have been a problem already before the population went extinct because the sets of genes that run the immune system and code for the proteins that protect the body from foreign molecules, the so-called major histocompatibility complex, had already been observed to lack variability. Then of course, in the case of the extinct Pyrenean ibex there is also another problem that would be a major impediment in the reconstruction of a natural population. It is a problem of the missing Y-chromosome. Goat Y-chromosomes would be necessary to have functional males of reconstructed goats. Basically this is a problem that has also puzzled thinkers with respect to the child that allegedly was born by a virgin on the solstice 2000 years ago. Did he have a chromosome set from the father?
Merry solstice, Christmas, and Happy New Year!
EW
More reading
Folch, J. et al. (2009) First birth of an animal from an extinct subspecies (Capra pyrenaica pyrenaica) by cloning. Theriogenology 71:1026–1034.
Gunnell, T. (1995) The Origins of Drama in Scandinavia. Boydell & Brewer Ltd.
Hinson, R. (2015) Goat. Reaction Books, London.
Lee, K. (2001) Can cloning save endangered species? Current Biology 11 (7): R245-R246
Today we present two more of Arne Nygrens gorgeous photos, that he made during our week in the field in Sletvik (central Norway). The subjects in both of these are polychaetes from the family Phyllodocidae, the paddleworms.
First up is a stunning Phyllodoce citrina collected from shell sand at about 60 m depth. The animal is approximately 6 cm long.
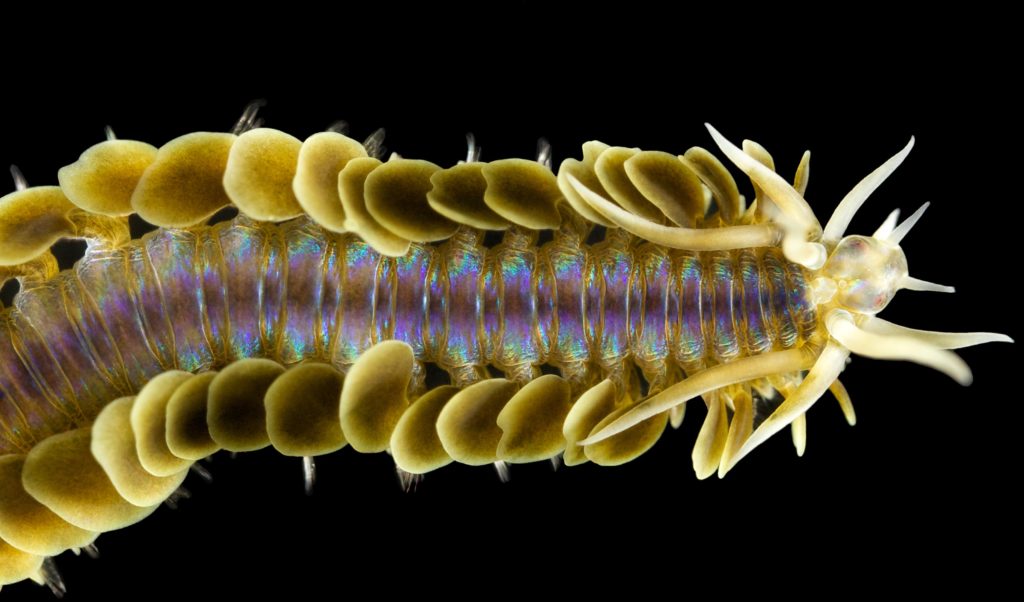
Phyllodoce citrina, Photo by Arne Nygren CC-BY-SA
The next one, Paranaitis sp. n. is actually a new species for science, which came as a pleasant surprise. This is a fairly well-studied group, and the locality Galgenes is one that has been sampled regularly – yet there it was! It is rather unusual to find species where one can so immediately recognize that they are something new; usually we need many specimens, and a combination of detailed studies of morphology and genetic work – but this one is possible to distinguish straight from morphology, as it was lacking eyes. The specimen is about 1.5 cm long.

Paranaitis n sp Photo by Arne Nygren CC-BY-SA
-Arne & Katrine
Last year we had a calendar post about the Heart of the Museum – our type collections.
To recap, a species’ type is “…the objective standard of reference for the application of zoological names. When a new species or subspecies is described, the specimen(s) on which the author based his/her description become the type(s) (Article 72.1). In this way names are linked to type specimens, which can be referred to later if there is doubt over the interpretation of that name.
Consequently types are sometimes referred to as “onomatophores” which means name bearers.”
– International Commission on Zoological Nomenclature (IZN)
The location – sampling site – from which the type specimen is described is known as the type locality.
As you have probably noticed, polychaetes (bristle worms) are a focus group in our lab, and several species have type localities close by.
The biologist and theologian Michael Sars (1805-1869) lived in the Bergen region for many years. He was a prolific taxonomist, naming 277 species of marine taxa according to the World Register of Marine Species (WoRMS).
Consequently there are quite a few species that have their type locality within easy daytrip-distance by ship for us.
One such locality is Glesvær, where Michael Sars described several new species in his work of 1835: Beskrivelser og Iagttagelser over nogle mærkelige eller nye i Havet ved den Bergenske Kyst levende Dyr af Polypernes, Acalephernes, Radiaternes, Annelidernes og Molluskernes Classer* (“Descriptions and Observations of some strange or new animals found off the coast of Bergen, belonging to the Classes …”).
The polychaete Amphicteis gunneri (Ampharetidae) is one of these species. It was first described by Michael Sars as Amphitrite gunneri (the species name is an homage to Johan Ernst Gunnerus (1718-1773) who was an active scientist within botany and zoology, as well as the bishop in Trondheim, and one of the founders of Det Kongelige Norske Videnskapers Selskap) in the publication above. Here are his original illustrations of the species:
We have previously submitted several specimens of Amphicteis gunneri for DNA-barcoding through the NorBOL-project – and found that specimens that according to the keys in the literature should all come out nicely as A. gunneri in fact end up in several barcode-based groupings (BINs), meaning that they genetically different from each other. Then we need to unravel which one is the true A. gunneri, and decide what to do with the others. In such cases, material from type localities is invaluable. By sending in specimens identified by resident taxonomists as A. gunneri from the type locality, we hope to figure out which BIN represent A. gunneri, and which represent potentially new species.
We were also able to photograph live specimens showing the nice coloration of this worm. Fixed specimens lose this colour and become uniformly yellow/white (no dots).
*Thanks to the excellent Biodiversity Heritage Library, this publication can be found in full text online, accessible for everyone – go here to see it. The Flickr stream of BHL is also an excellent source of amazing illustrations, you can find that here.
-Tom & Katrine
Congratulations to Jenni, our (former!) master student, who presented her MSc project last Friday!
She has been working on the phylogenetic systematics and evolution of a genus of small marine gastropods called Phanerophthalmus, and she’s done an impressive amount of work.
The project was titled
Systematics, biogeography, and trophic ecology of the genus
Phanerophthalmus A. Adams, 1850 (Mollusca, Cephalaspidea, Haminoeidae) in
the Indo-West Pacific, and was supervised by Manuel Malaquias.

Celebrating our freshly minted MSC (second from the left in top photo) with coffee, cake and bubbles!
We wish you all the best, Jenni!
Polina Borisova, a first year master student from the Zoological Department of the Moscow State University (Russia), is coming to the Invertebrate Collections of the University Museum of Bergen with a 1-month research visit in January 2017.
Polina is going to work on the bristle worms from the family Lumbrineridae studying the collection from West Africa and Norway. Her project is jointly supervised by Dr. Nataliya Budaeva from the University Museum of Bergen and Dr. Alexander Tzetlin from the Moscow University.
Lumbrineridae are the worms with relatively poor external morphology but complex jaw apparatus. The structure of jaws has been traditionally used in the systematics of the family in the generic diagnoses. Polina is utilizing the methods of microCT to study the jaws of lumbrinerids in 3D.
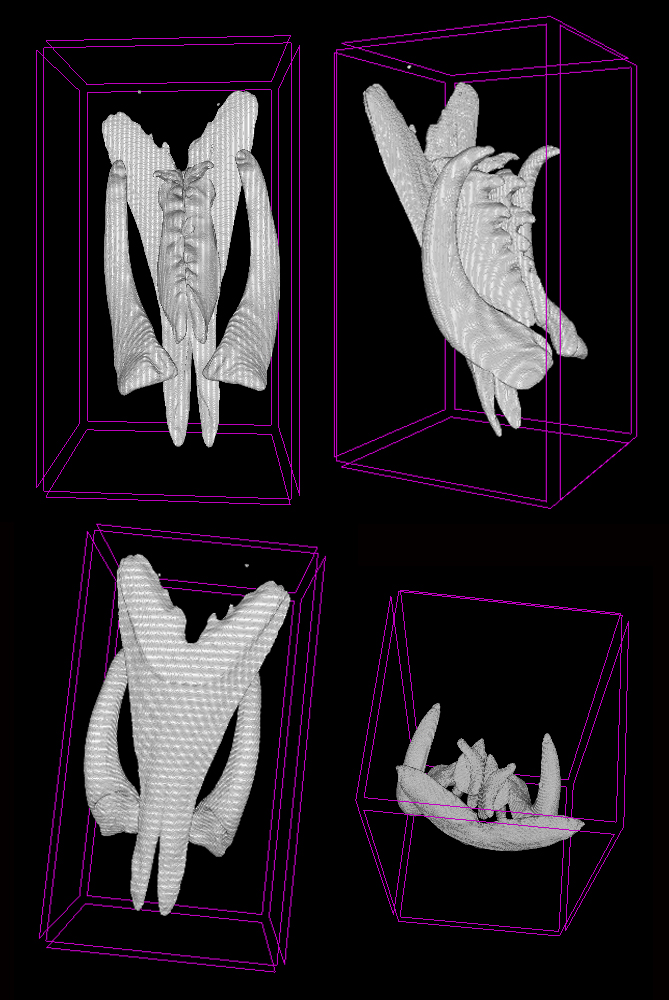
Jaws of Scoletoma fragilis from the White Sea scanned using microCT showing ventral solid mandibles, forceps-like maxillae I and denticulate maxillae II and II, carriers of maxillae are omitted (Photo: P. Borisova)
Polina is also going to sequence several genetic markers to reconstruct the first molecular phylogeny of the family. This will allow testing the current hypothesis on the intergeneric relationships within Lumbrineridae and will aid in tracing the evolution of jaws within the family.
-Nataliya & Polina
Molluscs come in a variety of shapes and sizes, but some of the least known are perhaps the Aplacophora, or shell-less molluscs. Instead of a shell, these worm-shaped molluscs have a cuticle covered in calcareous spicules, or sclerites, that give them a beautiful, glistening appearance!
The very first species of aplacophoran mollusc, Chaetoderma nitidulum, was collected from the Swedish west coast and described by the Swedish taxonomist Sven Lovén in 1844. At the time, it was not even known what animal group the new, strange animal belonged to. It had spicules– could it be related to the spiny sea urchins? It had a worm-like body– could it be related to other worm-shaped animals? It would be almost 50 years before it was conclusively recognized as part of Mollusca. Since then, many more species have been discovered, and today close to 500 species of aplacophoran molluscs have been described.
Chaetoderma nitidulum is known today as one of the common aplacophoran molluscs in the East Atlantic, with a distribution from the Svalbard archipelago in the north, to the British Isles in the south. However, taxonomist have been debating the identity of Chaetoderma nitidulum since shortly after it was described. Some researchers have suggested that it could in fact consist of up to six different species. Other researchers have synonymized it with other species, or suggested that it is not a separate species, but only part of a larger species which has a distribution that spans the entire North Atlantic.
The shape, size and the patterns on the calcareous sclerites covering the body of the aplacophoran molluscs is unique to each species, making it one of the most important characters we have to distinguish between different species.
Looking at the sclerites through the microscope equipped with a cross-polarizing filter gives us a shiny, colorful view of the sclerites. The light shines with different colors depending on the thickness of the sclerites, helping us get a good view of the structure of the sclerites.
We have recently investigated specimens of Chaetoderma nitidulum from different localities from the entire distribution range of the species. Our investigations have revealed a lot of variation between the specimens, both in the calcareous sclerites and in DNA sequences, separating the specimens into at least two different groups. Could it be that Chaetoderma nitidulum actually represents more than one species?
-Nina
Yesterdays door of this calendar introduced the bioluminescent animals of the deep sea.
In the parts of the ocean where sunlight reaches (the photic zone), production of ones own light is not common. This is because it is costly (energetically), and when the surroundings already are light, the effect is almost inexistent. An exception to this is the use of counter-illumination that some animals have: lights that when seen from underneath the animal camouflages them against the downwelling light from above.
But what then with the ocean during the polar night? Last Thursdays blog told the story of the dark upper waters during the constant dark of the arctic winter, and how the quite scanty light of the moon is enough to initiate vertical mass movements. Another thing we see in the dark ocean is that processes that at other latitudes are limited to the deep sea come up nearly to the surface during the polar night.
So – in the Arctic winter we don´t have to use robots and remote cameras to observe biioluminescent animals: we can often observe them using normal sport diving equipment or even from above the surface. A very recent study (Cronin et al, 2016) has measured the light from different communities in the Kongsfjord of Svalbard during the polar night. They found that going from the surface and down, dinoflagellates produced most light down to 20-40 m depth, the lighting “job” was then in general taken over by small copepods (Metridia longa). Most light was produced around 80 m depth.

Bioluminescent dinoflagellates shining through the winter sea ice in Kongsfjorden. Photo: Geir Johnsen, NTNU
It is possible to recognise different species from the light they make; a combination of the wavelength, the intensity and the length of the light-production gives a quite precise “thumbprint”. If we know the possible players of the system in addition, an instrument registering light will also be able to give us information about who blinks most often, at what depths, etc. Cronin and her coauthors have made a map of the lightmakers in the Kongsfjord.
This is all well and good, but the next question is of course WHY. There can be several uses for light, and we can bulk the different reasons into 3 main groups: Defense, offense and recognition.

Different strategies for Bioluminescence. Fig 7 from Haddock (2010), redrawn for representation of the Polar night bioluminescence by Ola Reibo for the exhibition “Polar Night”

The bioluminescent cloud from an escaping krill. Kongfjorden, during the Arctic polar night. Photo: Geir Johnsen, NTNU
Defence has already been mentioned above: the counterillumination against downwelling light is helping an animal defend itself against predation. Some will leave a smokescreen, or even detach a glowing bodypart while swimming away in the dark, and others blink to startle the enemy or to inform their group-mates that an enemy is getting close.
Offense is mainly to use the light to get food (this is typical angler-fish-behaviour), and recognition is very often about flirting. Instead of flashing your eyelashes at your your chosen potential partner, you flash some light at him or her…
Thursdays are about amphipods in this blog, so here they come. Bioluminescent amphipods are present mainly in the hyperiid genera Scina (a Norwegian representative of this genus is Scina borealis (Sars, 1883).) Hyperiids are amphipods that swim in the free watermasses, like most other bioluminescent animals.
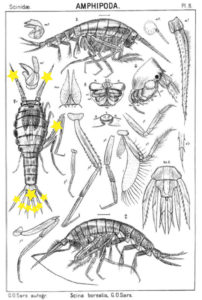
The bioluminescent amphipod Scina borealis (Sars, 1893). The added stars indicate where the bioluminescence occurs. Original figure: G.O.Sars, 1895.
Crustacea use more different ways to produce bioluminescence than most other groups – this points to a possibility that the use of bioluminescence has evolved several independent times in this group. So the copepod Metridia longa will use a different chemical reaction than the krill, and the amphipods use again (several) different reactions. Some research on the bioluminescence of amphipods was undertaken already in the late 1960s, where P Herring collected several Scina species and kept them alive in tanks. There he exposed them to several luminescence-inducing chemicals and to small electrical shocks, to see where on the body light was produced and in what sort of pattern. He reported that Scina has photocytes (lightproducing cells) on the antennae, on the long 5th “walkinglegs”, and on the urosome and uropods. They would produce a nonrythmical rapid blinking for up to 10 seconds if attacked, and at the same time the animal would go rigid in a “defence-stance” with the back straight, the antennae spread out in front of the head, and the urosome stretched to the back. This definitely seems to be a defence-ligthing, maybe we should even be so bold as to say it would startle a predator?
Anne Helene
Literature:
Cronin HA, Cohen JH, Berge J, Johnsen G, Moline MA (2016) Bioluminescence as an ecological factor during high Arctic polar night. Scientific Reports/Nature 6, article 36374 (DOI: 10.1038/srep36374)
Haddock SHD, Moline MA, Case JF (2010) Bioluminescence in the Sea. Annual Review of Marine Science 2, 443-493
Herring PJ (1981) Studies on bioluminescent marine amphipods. Journal of the Marine biological Association of the United Kingdoms 61, 161-176.
Johnsen G, Candeloro M, Berge J, Moline MA (2014) Glowing in the dark: Discriminating patterns of bioluminescence from different taxa during the Arctic polar night. Polar Biology 37, 707-713.